Question:
Give answer in 3 steps with explanation at the and of each step and final answer at the end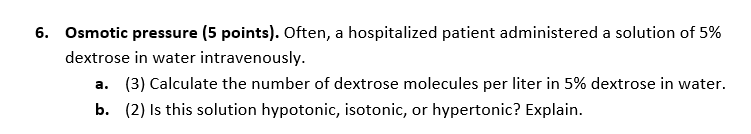 6. Osmotic pressure (5 points). Often, a hospitalized patient administered a solution of 5%
dextrose in water intravenously.
a. (3) Calculate the number of dextrose molecules per liter in 5% dextrose in water.
b. (2) Is this solution hypotonic, isotonic, or hypertonic? Explain.
6. Osmotic pressure (5 points). Often, a hospitalized patient administered a solution of 5%
dextrose in water intravenously.
a. (3) Calculate the number of dextrose molecules per liter in 5% dextrose in water.
b. (2) Is this solution hypotonic, isotonic, or hypertonic? Explain.
This is AI generated Response.
Asked by: Test User 100162
Created at: 2025-05-07 07:51:17
Asked by: Test User 100162
Created at: 2025-05-07 07:51:17
Answer:
Rendered
Raw Text
Let's solve each part step by step with explanations:
---
### 6a. Calculate the number of dextrose molecules per liter in 5% dextrose in water.
#### **Step 1: Determine the mass of dextrose in 1 liter**
- A 5% (w/v) solution means 5 grams of dextrose in 100 mL of water.
- So, in 1 liter (1000 mL):
\( 5 \text{ g} \times 10 = 50 \text{ g (dextrose per liter)} \)
**Explanation:**
We multiplied by 10 because 1 L = 1000 mL and 1000/100 = 10.
---
#### **Step 2: Convert grams to moles**
- Molar mass of dextrose (glucose, C₆H₁₂O₆) ≈ **180 g/mol**
- Moles of dextrose per liter:
\( \frac{50 \text{ g}}{180 \text{ g/mol}} = 0.278 \text{ mol/L} \)
**Explanation:**
We use the formula: moles = mass / molar mass.
---
#### **Step 3: Calculate the number of molecules**
- 1 mole = \( 6.022 \times 10^{23} \) molecules
- Number of molecules per liter:
\( 0.278 \text{ mol} \times 6.022 \times 10^{23} \text{ molecules/mol} \approx 1.67 \times 10^{23} \text{ molecules} \)
**Explanation:**
We multiply the number of moles by Avogadro's number to get the number of molecules.
---
**Final Answer for 6a:**
There are approximately **\( 1.67 \times 10^{23} \) dextrose molecules per liter** in a 5% dextrose solution.
---
### 6b. Is this solution hypotonic, isotonic, or hypertonic? Explain.
#### **Step 1: Understand isotonicity**
- An isotonic solution has the same osmolarity as blood plasma (~0.9% NaCl or ~300 mOsm/L).
**Explanation:**
Isotonic solutions do not cause cells to shrink or swell.
---
#### **Step 2: Compare 5% dextrose to isotonic solutions**
- 5% dextrose is considered **isotonic** when administered, but it becomes **hypotonic** after dextrose is metabolized (since only water remains).
**Explanation:**
Initially, 5% dextrose has similar osmolarity to plasma, but as cells take up and metabolize glucose, only water is left, diluting the blood.
---
**Final Answer for 6b:**
**5% dextrose in water is considered isotonic when administered intravenously, but it acts as a hypotonic solution after metabolism of dextrose.**
This is because the initial osmolarity matches that of plasma, but as the glucose is used up, only water remains, making the solution hypotonic relative to cells.
Question:
final answer in short
This is AI generated Response.
Asked by: Test User 100162
Created at: 2025-05-07 08:00:45
Asked by: Test User 100162
Created at: 2025-05-07 08:00:45
Answer:
Rendered
Raw Text
**6a.** There are approximately **\(1.67 \times 10^{23}\)** dextrose molecules per liter in 5% dextrose solution.
**6b.** The solution is **initially isotonic** with blood plasma but becomes **hypotonic** after dextrose metabolism.
📢 Instructions:
- Ask anything relatd to above question only.
- For new Question goto :